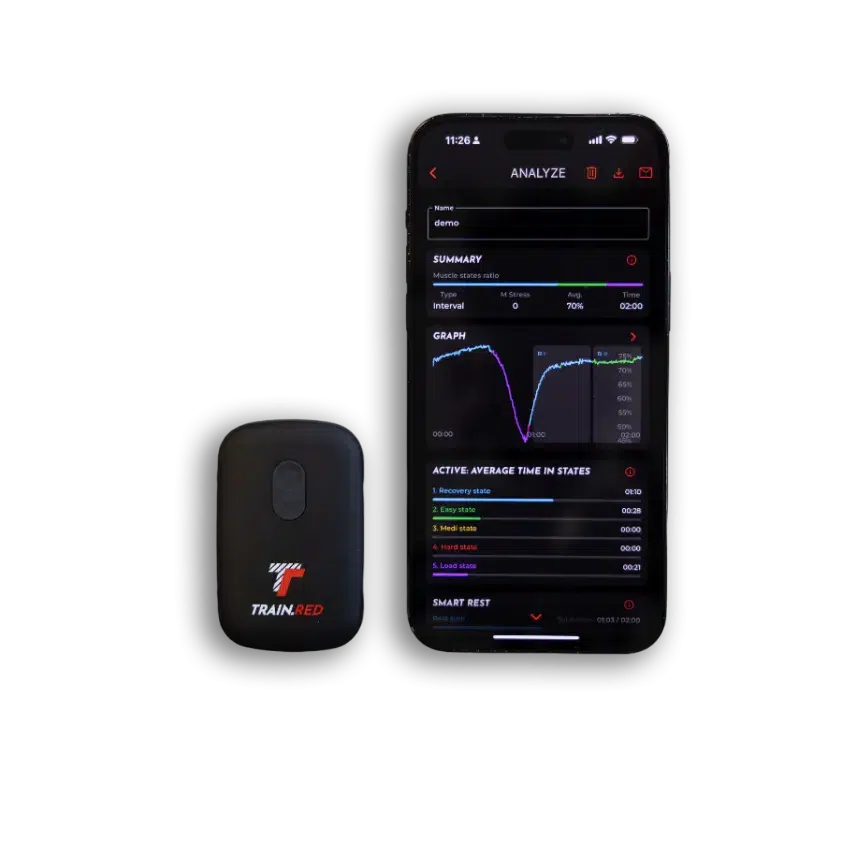
When you move, your muscles need energy. They get this energy by breaking something called ATP. To make ATP in the best way, your muscles need oxygen.
We Measure Oxygen in Your Muscles
Every cell in your body needs oxygen to work. Your body sends oxygen all around through your blood, so your cells can get what they need.
When you breathe in, oxygen sticks to a special part in your red blood cells. This oxygen travels through your blood to your muscles and other parts of your body.
When you exercise, your muscles need even more oxygen!
You might wonder, how can we know how much oxygen is in your muscles without opening your body? We use a special tool that shines light through your skin and measures how much oxygen your muscles have.
This tool is called Near-Infrared Spectroscopy (NIRS). It uses light that doesn’t hurt or bother you, just like shining a tiny flashlight through your finger. The tool helps us see how much oxygen is in your muscles and whether they’re getting the energy they need.
Exercise experts know how to read this data, but we want to make it easy for everyone to understand. We are always improving our technology to make it better.
Right now, we can tell you what your muscles are doing in 5 simple ways:
- Resting and recovering
- Working lightly
- Working at a steady, moderate pace
- Working really hard without enough oxygen (called anaerobic)
- Pushing your muscles harder and harder
Our smart system looks at changes in oxygen and tells you exactly what’s happening inside your muscles.
When you move, your muscles need energy. They get this energy by breaking something called ATP. To make ATP in the best way, your muscles need oxygen.
We Measure Oxygen in Your Muscles
Every cell in your body needs oxygen to work. Your body sends oxygen all around through your blood, so your cells can get what they need.
When you breathe in, oxygen sticks to a special part in your red blood cells. This oxygen travels through your blood to your muscles and other parts of your body.
When you exercise, your muscles need even more oxygen!
You might wonder, how can we know how much oxygen is in your muscles without opening your body? We use a special tool that shines light through your skin and measures how much oxygen your muscles have.
This tool is called Near-Infrared Spectroscopy (NIRS). It uses light that doesn’t hurt or bother you, just like shining a tiny flashlight through your finger. The tool helps us see how much oxygen is in your muscles and whether they’re getting the energy they need.
Exercise experts know how to read this data, but we want to make it easy for everyone to understand. We are always improving our technology to make it better.
Right now, we can tell you what your muscles are doing in 5 simple ways:
- Resting and recovering
- Working lightly
- Working at a steady, moderate pace
- Working really hard without enough oxygen (called anaerobic)
- Pushing your muscles harder and harder
Our smart system looks at changes in oxygen and tells you exactly what’s happening inside your muscles.



Hardcore NIRS
How Near Infrared Spectroscopy (NIRS) started
NIRS started with a paper published by Frans Jöbsis in Science (1977), Jöbsis reported that biological tissues are relatively transparent to light in the near infrared (700-1300 nm) region. Therefore, it is possible to transmit enough photons through organs for in situ monitoring. In this near infrared region, hemoglobin - including its two main variants oxyhemoglobin (O2Hb) and deoxyhemoglobin (HHb)- exhibits oxygen-dependent absorption. Hemoglobin is assumed to be the main chromophore in biological tissue that absorbs light in this near infrared region.
The science
If the absorption is known, the Beer-Lambert law can be used to calculate the chromophore's absorption. The Lambert-Beer law is given by: ODλ = Log (I0/I) = ελ * c * L
ODλ is a dimensionless factor known as the optical density of the medium, I0 is the incident light, I the transmitted light, ελ the chromophore's extinction coefficient (in µM-1•cm-1), c is the concentration (in µM) of the chromophore, L the distance (in cm) between light entry and exit points and λ is the wavelength used (in nm).
The Beer-Lambert law is intended to be used in a transparent, non-scattering medium. When it is applied to a scattering medium , e.g. biological tissue, a dimensionless pathlength correction factor must be incorporated. This factor, sometimes called the differential pathlength factor (DPF), accounts for the increase in optical pathlength due to scattering in the tissue. The modified Beer-Lambert law for a scattering medium is given by: Δc = ΔODλ / (ελ * L * DPF)
Where ODλ represents the oxygen-independent optical losses due to scattering and absorption in the tissue. Assuming that ODλ is constant during a NIRS measurement, we can convert the change in optical density into a change in concentration.
This equation is valid for a medium with one chromophore. If more chromophores are involved, we need to measure at least as many wavelengths as there are chromophores present. This results in a set of linear equations. The solution of this set leads to the algorithm used in most NIRS systems. A scattering medium makes it possible to
measure the absorption with the near infrared source and detector parallel to each other. This offers the opportunity to measure oxygenation in larger tissues, e.g. muscles and brain using NIRS equipment.
NIRS algorithm
Defining the algorithm used by NIRS requires the spectral extinction coefficients of the various chromophores. The spectra of the two main chromophores, O2Hb and HHb.
The sum of O2Hb and HHb is a measure of the total blood volume (tHb) in the tissue. Muscle tissue contains two further chromophores: oxy- and deoxymyoglobin (O2Mb and HMb). In order to distinguish between hemoglobin and myoglobin in muscle tissue, the spectra need to be sufficiently different. Unfortunately this is not the case in the near infrared region of the spectrum. This means, NIRS cannot distinguish if the measured oxygen concentration is carried by hemoglobin or myoglobin. The wavelengths which can distinguish the Hb and Mb are not able to penetrate the tissue deep enough.
FREQUENTLY ASKED QUESTIONS
What’s the difference between NIRS and pulse oximetry?
The technique on which near infrared spectroscopy relies is closely analogous to the technique of pulse oximetry.
The main difference is the tissue being sampled. Pulse oximetry calculates the percentage of oxygenated hemoglobin in the arterial blood. NIRS calculates the changes in oxy- and deoxyhemoglobin (and optionally the percentage of oxygenated hemoglobin) in the tissue under investigation (capillaries), which contains both arterial and venous blood.
more questions
It depends on where you are. Orders processed here will take 5-7 business days to arrive. Overseas deliveries can take anywhere from 7-16 days. Delivery details will be provided in your confirmation email.
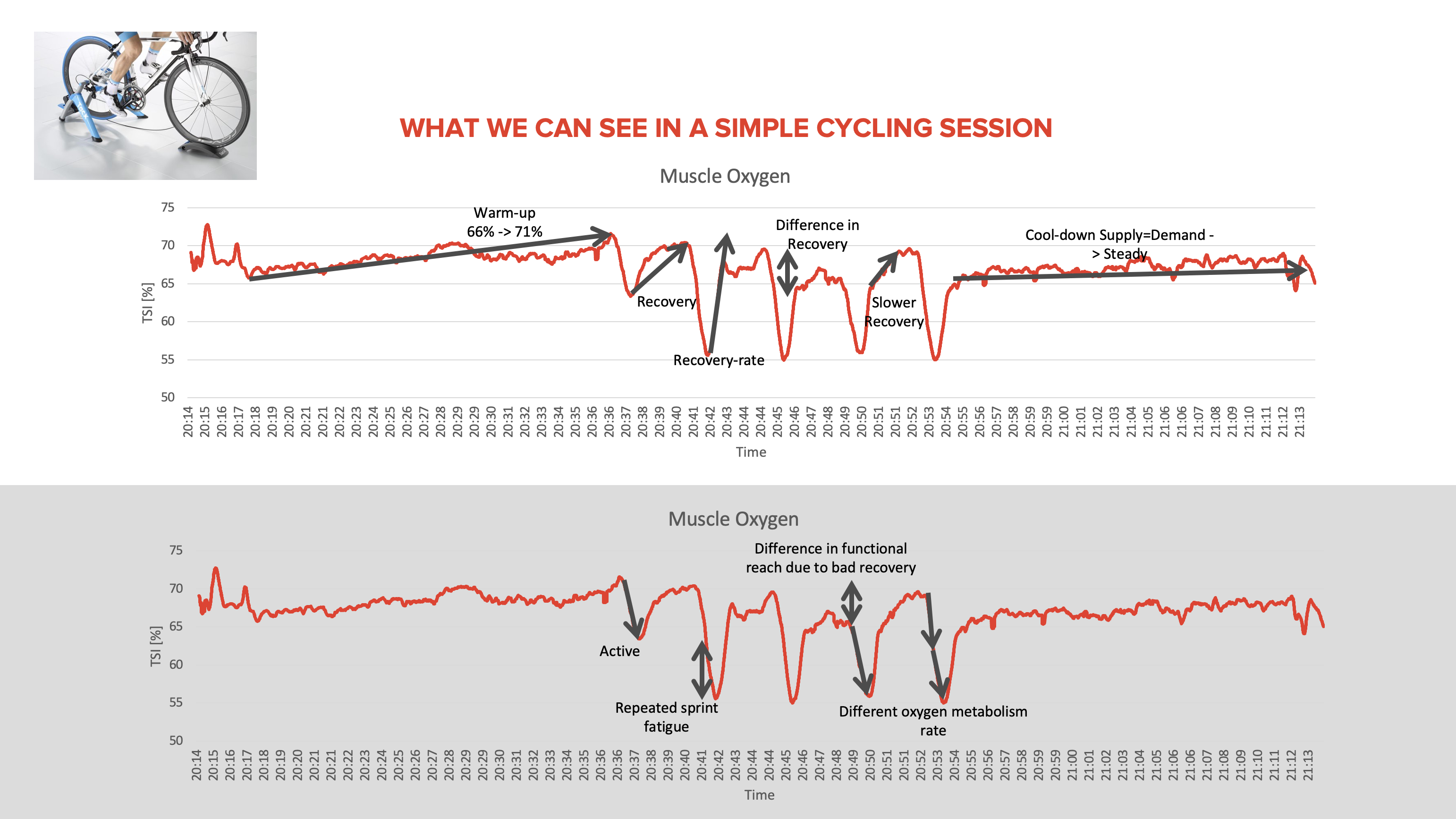
More Science
For your convenience, our mother company Artinis has compiled a list of all (f)NIRS-literature performed with our equipment.
All publications